-
Promotion
Special Promotion
-
Products
Sectors
Categories
Product Information
- How to order
- Apostle Catalog - 2024 April (PDF)
- MiniMax: Best-in-class gDNA/cfDNA Isolation Technology (PDF)
- MagTouch 3000: World 1st Large-Volume Fully-Automated cfDNA Extractor (PDF)
- Best-in-Class total-RNA/cfRNA Isolation Technology (PDF)
- Cost-efficient & High-quality Laboratory Consumables (PDF)
-
Technology
- Apostle MiniMax Technology
- Apostle MiniMax (cfRNA)
- Apostle MagTouch Technology
- Apostle BCT Technology
- Apostle MiniEnrich Technology
- Apostle MiniGenomics Technology
- Apostle Triton Technology
- Apostle MiniMax (Type S)
- Apostle Viral RNA/DNA Isolation Automation System
- Apostle Well Plates and Accessories for Nucleic Acids Isolation
- Applications
- Sign In
Why Apostle
Laboratory costs are high. Apostle supports our community with high-quality, cost-effective, and eco-friendly products:
That’s why more and more research and clinical laboratories are now switching to Apostle technologies.
- Superior performance
- Minimum 50% of savings on cost
- Top-notch technical support
- Environmental-friendly. Save mother nature.
That’s why more and more research and clinical laboratories are now switching to Apostle technologies.
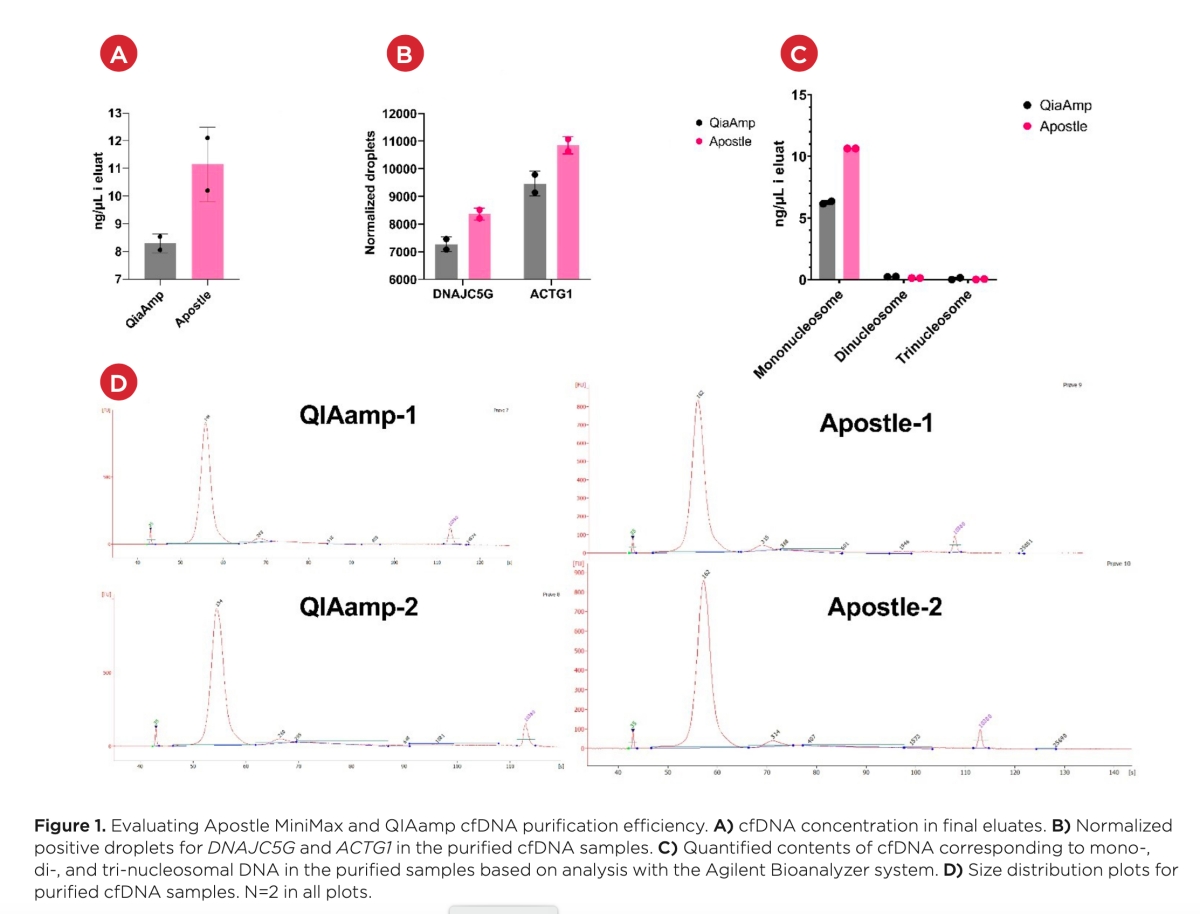
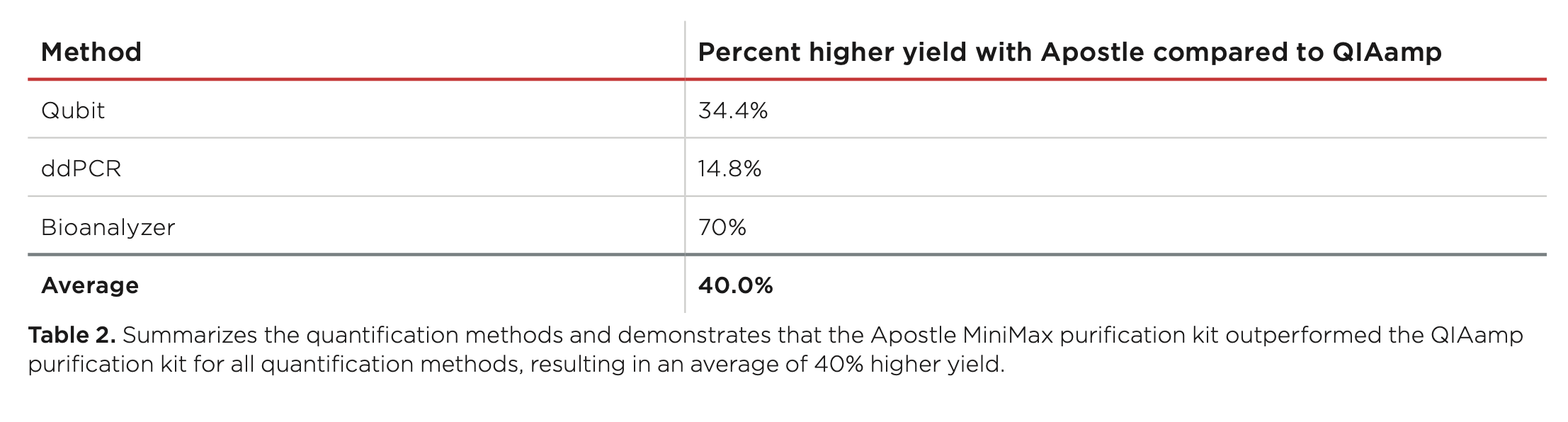
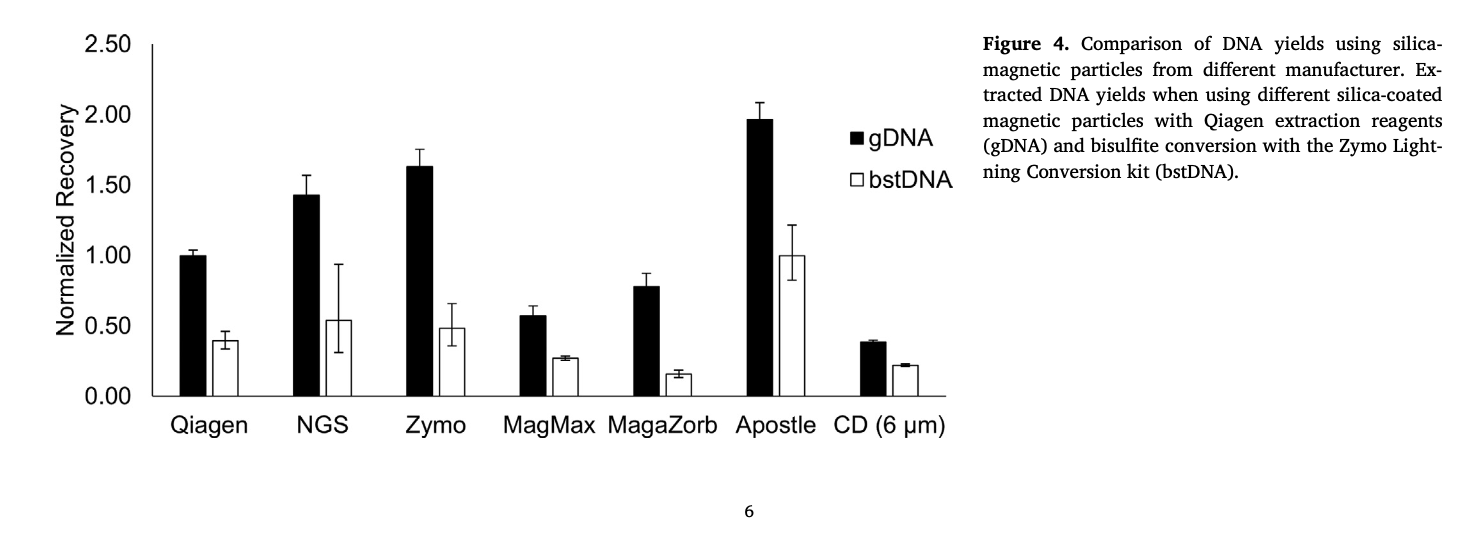
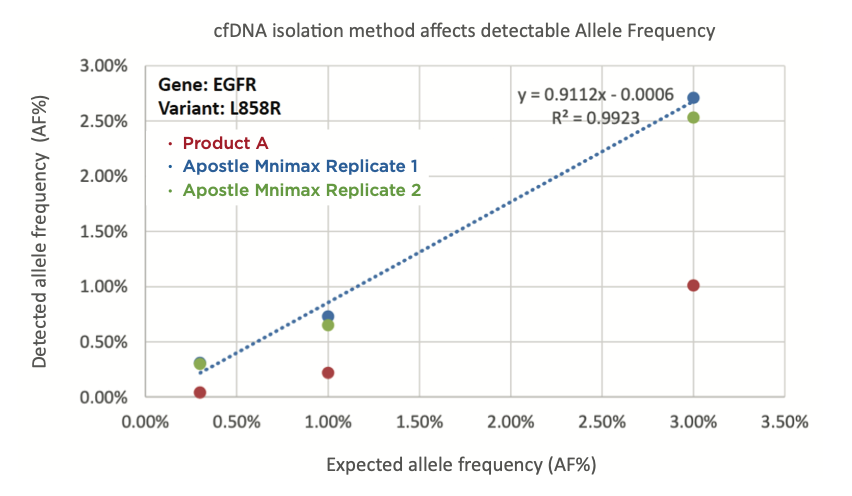
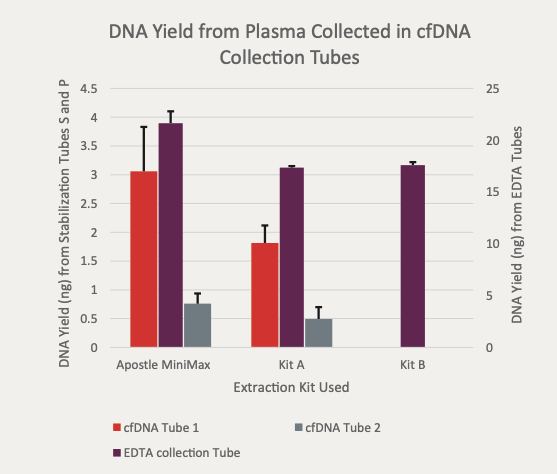
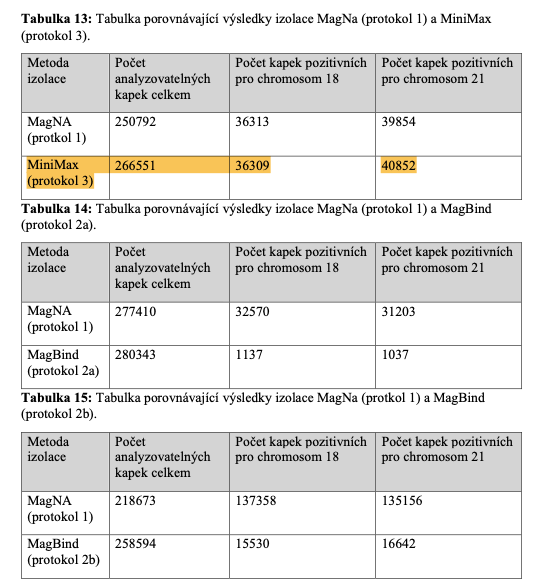
Best-in-Class Performance in cfDNA Isolation: Side by Side Comparison
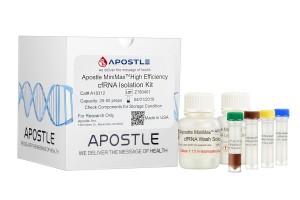
Apostle MiniMax High Efficiency Cell-Free DNA Isolation Kit
The ability to isolate and analyze circulating cell free DNA (cfDNA) at very low concentrations is becoming increasingly important, particularly in non-invasive prenatal test (NIPT), early cancer detection, and infectious disease diagnosis. Highly efficient isolation of cfDNA from complexed biological medium is a crucial step for subsequent cfDNA analysis.
Apostle MiniMax technology offers a best-in-class efficiency and purity compared with conventional technologies to capture and isolate the circulating cell-free genetic materials. Its superb performance has been extensively validated.
As used in:
As used in:
- Integrative modeling of tumor genomes and epigenomes for enhanced cancer diagnosis by cell-free DNA. Nature Communications 2023;14:2017
- Simultaneous analysis of mutations and methylations in circulating cell-free DNA for hepatocellular carcinoma detection. Science Translational Medicine 2022;14:672
- Individualized, heterologous chimpanzee adenovirus and self-amplifying mRNA neoantigen vaccine for advanced metastatic solid tumors: phase 1 trial interim results. Nature Medicine 2022;28: 1619-1629
- Safety and tolerability of AAV8 delivery of a broadly neutralizing antibody in adults living with HIV: a phase 1, dose-escalation trial. Nature Medicine 2022; 28:1022-1030
- Efficient detection and post-surgical monitoring of colon cancer with a multi-marker DNA methylation liquid biopsy. PNAS 2021;118 (5) e2017421118
- More ...
MagTouch 3000
Apostle MagTouch 3000 is world's first large volume nucleic acid extraction automation system that is capable of processing up to 6-10 mL of liquid biological samples in one single well. It completely eliminates the needs to split samples into 2 mL aliquots like the traditional small-volume and semi-automated systems require.
Designed for fast extraction and purification of nucleic acids from large volume of liquid biological samples, for example plasma, serum, urine, etc, in 24 well format.
Designed for fast extraction and purification of nucleic acids from large volume of liquid biological samples, for example plasma, serum, urine, etc, in 24 well format.
- Large Volume: capable of processing up to 6-10 mL of liquid biological samples in one single well.
- Fully Automated and Fast: One button start; Requires no human intervention during the run; Run time is approximately 2 hours per 24 large-volume samples.
- High Efficiency and Low Cost: Guaranteed 50% improved efficiency and reduced cost, compared with the traditional small-volume and semi-automated systems.
- Safer: Integrated with a UV light for unit interior disinfection.
Apostle MiniMax High Efficiency Cell-Free RNA Isolation Kit
Apostle MiniMax High Efficiency cfRNA Isolation Kit offers superior isolation efficiency of cell-free RNAs between 17 nt to 1000 nt, without phenol or chloroform. it is ready for a broad range of subsequent applications, including sequencing, PCR, etc. It is suitable for processing samples collected in various major blood collection tubes, especially which will prevent RNA degradation during storage.
- As used in: Terminal modifications independent cell-free RNA sequencing enables sensitive early cancer detection and classification. Wang et al. Nature Communications 15, Article number: 156 (2024).
- More ...
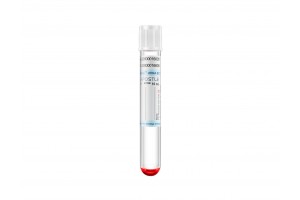
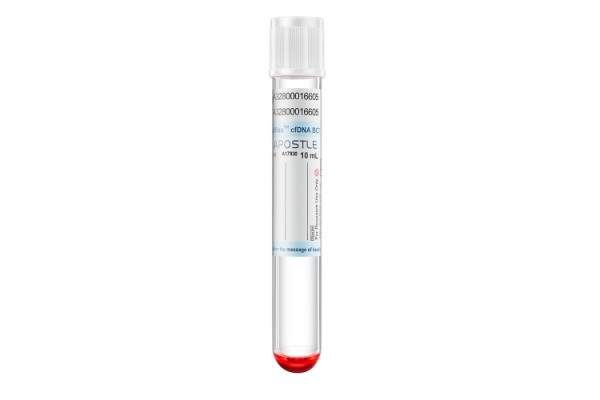
Apostle MiniMax Cell-Free DNA Blood Collection Tube
Powered by Apostle MiniMax technology, Apostle MiniMax cfDNA Blood Collection Tube (BCT) is an excellent tool for blood cfDNA preservation during blood collection, storage and transport.
This is achieved through Apostle MiniMax cfDNA BCT’s ability to:
This is achieved through Apostle MiniMax cfDNA BCT’s ability to:
- Prevent the release and contamination of genomic DNA from cells in blood during storage and transportation.
- Preserve existing cfDNA in blood from degradation.
- Prevent existing cfDNA in blood from cross-linking with with other biomolecules (i.e. protein).
- As used in: SneakPeek Test
- More ...
Apostle MiniEnrich Short Fragments Enrichment Kit
Precise DNA sizing can boost sequencing efficiency, reduce cost, improve data quality, and even allow sequencing of low-input samples, while current pervasive DNA sizing approaches are incapable of differentiating DNA fragments under 200 bp with high resolution (<20 bp). The Apostle MiniEnrich Short Fragments Enrichment Kit is a simple, automatable, high-resolution DNA size enrichment workflow on a magnetic nano-platform to exploit this 20 bp size difference and to enrich fetal DNA fragments from maternal blood.
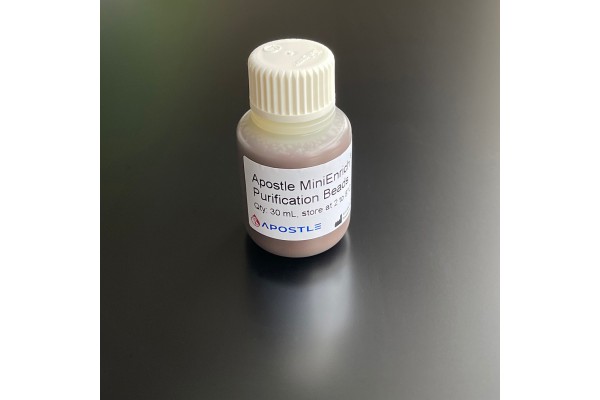
Apostle MiniEnrich Carboxyl Beads for Purification (AMPure XP alternative)
Next-generation purification, size selection, and long-read size selection.
The Apostle MiniEnrich Purification Beads is designed for purification and clean-up of DNA fragments from the contaminants in NGS and molecular biology workflows. It can be used on samples after DNA isolation, fragmentation, PCR amplification, cloning, library preparation, etc. With proprietary Apostle MiniEnrich technology, the kit is featured for its more efficient removal of contaminants and recovery of DNA fragments of interest than current market-leading products. This product is an effective alternative to AMPure XP, a brand name owned by Beckman Coulter. This product is not manufactured by Beckman Coulter.
The Apostle MiniEnrich Purification Beads is designed for purification and clean-up of DNA fragments from the contaminants in NGS and molecular biology workflows. It can be used on samples after DNA isolation, fragmentation, PCR amplification, cloning, library preparation, etc. With proprietary Apostle MiniEnrich technology, the kit is featured for its more efficient removal of contaminants and recovery of DNA fragments of interest than current market-leading products. This product is an effective alternative to AMPure XP, a brand name owned by Beckman Coulter. This product is not manufactured by Beckman Coulter.
What our community say
Twitter / X
News
February 1, 2024
About Us
Apostle Inc is a biotechnology company in Pleasanton, CA and San Jose, CA, a provider of innovative technologies and services for public health and life sciences. Apostle develops and provides best-in-class nucleic acid isolation and preservation technologies, including innovative technologies in the space of liquid biopsy - the sampling and analysis of non-solid biological tissue, primarily blood, often utilizing circulating free DNA (cfDNA) as a biomarker. Apostle technologies have been applied in many world-class R&D studies, clinical laboratory settings, and public health response and surveillance. To date, over 20 million samples have been successfully processed using our technologies, and the number is quickly growing.